- Avogadro's Principle Example
- Avogadro Real Life Example
- Avogadro's Law Example Problem
- Avogadro's Law Formula Example
You have learned about Avogadro's hypothesis: equal volumes of any gas at the same temperature and pressure contain the same number of molecules. It follows that the volume of a gas is directly proportional to the number of moles of gas present in the sample. Avogadro’s Law Examples in Real Life Avogadro’s law tells about the relationship between the volume of a gas and the number of molecules possessed by it. It was formulated by an Italian scientist Amedeo Avogadro in the year 1811. Formula of Avogadro’s Number. The numbers of atoms required are such that the number of grams of a substance turns out to be equal to the substance’s atomic mass. N A = 6.0220 x 10 23 mol-1. The word mole refers to the Avogadro’s number of a substance. For example, a mole of carbon-12 atoms happens to be 12 grams.
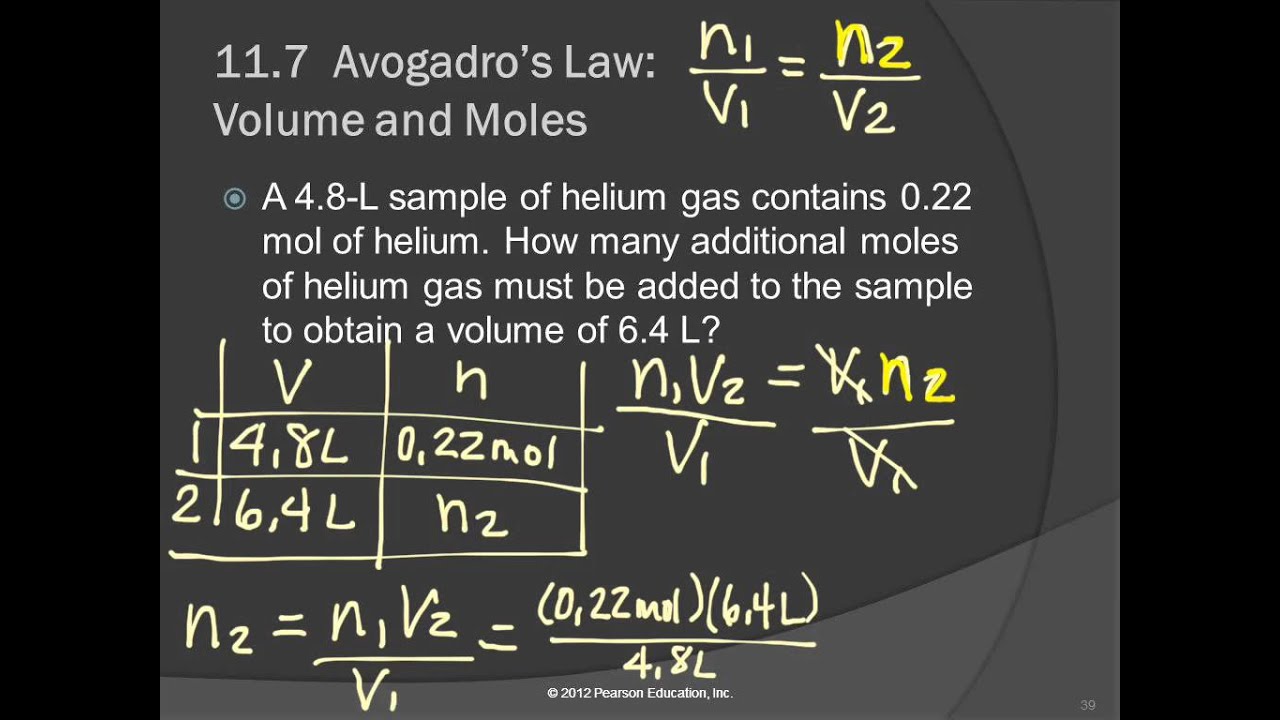
Question: Three balloons are filled with different amounts of an ideal gas. One balloon is filled with 3 moles of the ideal gas, filling the balloon to 30 L.
a) One balloon contains 2 moles of gas. What is the volume of the balloon?
b) One balloon encloses a volume of 45 L. How many moles of gas are in the balloon?
Answer:
Avogadro’s law says the volume (V) is directly proportional to the number of molecules of gas (n) at the same temperature.
n ∝ V
This means the ratio of n to V is equal to a constant value.
Since this constant never changes, the ratio will always be true for different amounts of gas and volumes.
where
ni = initial number of molecules
Vi = initial volume
nf = final number of molecules
Vf = final volume.
Part a) One balloon has 3 moles of gas in 30 L. The other has 2 moles in an unknown volume. Plug these values into the above ratio:
Solve for Vf
(3 mol)Vf = (30 L)(2 mol)
(3 mol)Vf = 60 L⋅mol
Vf = 20 L
You would expect less gas to take up a smaller volume. In this case, 2 moles of gas only took up 20 L.
Part b) This time, the other balloon has a known volume of 45 L and an unknown number of moles. Start with the same ratio as before:
Use the same known values as in part a, but use 45 L for Vf.
Solve for nf
(3 mol)(45 L) = (30L)nf
135 mol⋅L = (30L)nf
nf = 4.5 moles
The larger volume means there is more gas in the balloon. In this case, there are 4.5 moles of the ideal gas in the larger balloon.
An alternative method would be to use the ratio of the known values. In part a, the known values were the number of moles. There was the second balloon had 2/3 the number of moles so it should have 2/3 of the volume and our final answer is 2/3 the known volume. The same is true of part b. The final volume is 1.5 times larger so it should have 1.5 times as many molecules. 1.5 x 3 = 4.5 which matches our answer. This is a great way to check your work.
Avogadro Number Calculations II
How Many Atoms or Molecules?
The value I will use for Avogadro's Number is 6.022 x 1023 mol¯1.
Types of problems you might be asked look something like these:
0.450 mole of Fe contains how many atoms? (Example #1)0.200 mole of H2O contains how many molecules? (Example #2)
0.450 gram of Fe contains how many atoms? (Example #3)
0.200 gram of H2O contains how many molecules? (Example #4)
When the word gram replaces mole, you have a related set of problems which requires one more step.
And, two more:
0.200 mole of H2O contains how many atoms?
0.200 gram of H2O contains how many atoms?
When the word gram replaces mole, you have a related set of problems which requires one more step. In addition, the two just above will have even another step, one to determine the number of atoms once you know the number of molecules.
Here is a graphic of the procedure steps:
Pick the box of the data you are given in the problem and follow the steps toward the box containing what you are asked for in the problem.
Example #1: 0.450 mole of Fe contains how many atoms?
Solution:
Start from the box labeled 'Moles of Substance' and move (to the right) to the box labeled 'Number of Atoms or Molecules.' What do you have to do to get there? That's right - multiply by Avogadro's Number.0.450 mol x 6.022 x 1023 mol¯1 = see below for answer
Example #2: 0.200 mole of H2O contains how many molecules?
Solution:
Start at the same box as Example #1.0.200 mol x 6.022 x 1023 mol¯1 = see below for answer
The answers (including units) to Examples #1 and #2
The unit on Avogadro's Number might look a bit weird. It is mol¯1 and you would say 'per mole' out loud. The question then is WHAT per mole?
The answer is that it depends on the problem. In the first example, I used iron, an element. Almost all elements come in the form of individual atoms, so the correct numerator with most elements is 'atoms.' (The exceptions would be the diatomic elements plus P4 and S8.)
So, doing the calculation and rounding off to three sig figs, we get 2.71 x 1023 atoms. Notice 'atoms' never gets written until the end. It is assumed to be there in the case of elements. If you wrote Avogadro's Number with the unit atoms/mol in the problem, you would be correct.
The same type of discussion applies to substances which are molecular in nature, such as water. So the numerator I would use in example #2 is 'molecule' and the answer is 1.20 x 1023 molecules.
Once again, the numerator part of Avogadro's Number depends on what is in the problem. Other possible numerators include 'formula units,' ions, or electrons. These, of course, are all specific to a given problem. When a general word is used, the most common one is 'entities,' as in 6.022 x 1023 entities/mol.
Keep this in mind: the 'atoms' or 'molecules' part of the unit is often omitted and simply understood to be present. However, it will often show up in the answer. Like this:
0.450 mol x 6.022 x 1023 mol¯1 = 2.71 x 1023 atoms
It's not that a mistake was made, it's that the 'atoms' part of atoms per mole was simply assumed to be there.
Example #3: 0.450 gram of Fe contains how many atoms?
Example #4: 0.200 gram of H2O contains how many molecules?
Look at the solution steps in the image above and you'll see we have to go from grams (on the left of the image above) across to the right through moles and then to how many atoms or molecules.
Solution to Example #3:
Step One (grams ---> moles): 0.450 g / 55.85 g/mol = 0.0080573 molStep Two (moles ---> how many): (0.0080573 mol) (6.022 x 1023 atoms/mol) = 4.85 x 1021 atoms
Solution to Example #4:
Step One: 0.200 g / 18.015 g/mol = 0.01110186 molStep Two: (0.01110186 mol) (6.022 x 1023 molecules/mol) = 6.68 x 1021 molecules
Example #5: Calculate the number of molecules in 1.058 mole of H2O
Solution:
(1.058 mol) (6.022 x 1023 mol¯1) = 6.371 x 1023 molecules
Example #6: Calculate the number of atoms in 0.750 mole of Fe
Solution:
(0.750 mol) (6.022 x 1023 mol¯1) = 4.52 x 1023 atoms (to three sig figs)
Example #7: Calculate the number of molecules in 1.058 gram of H2O
Solution:
 (1.058 g / 18.015 g/mol) (6.022 x 1023 molecules/mole)
(1.058 g / 18.015 g/mol) (6.022 x 1023 molecules/mole)Here is the solution set up in dimensional analysis style:
| 1 mol | 6.022 x 1023 | |||
| 1.058 g x | ––––––––– | x | –––––––––– | = 3.537 x 1022 molecules (to four sig figs) |
| 18.015 g | 1 mol | |||
| ↑ grams to moles ↑ | ↑ moles to ↑ molecules | |||
Example #8: Calculate the number of atoms in 0.750 gram of Fe
(0.750 gram divided by 55.85 g/mole) x 6.022 x 1023atoms/mole/deflating-balloon-565093109-5794f9c65f9b58173bb1c4aa.jpg)
| 1 mol | 6.022 x 1023 | |||
| 0.750 g x | ––––––––– | x | –––––––––– | = 8.09 x 1021 atoms (to three sig figs) |
| 55.85 g | 1 mol |
Example #9: Which contains more molecules: 10.0 grams of O2 or 50.0 grams of iodine, I2?
Solution:
Basically, this is just two two-step problems in one sentence. Convert each gram value to its mole equivalent. Then, multiply the mole value by Avogadro's Number. Finally, compare these last two values and pick the larger value. That is the one with more molecules.
| 1 mol | 6.022 x 1023 | |||
| 10.0 g x | ––––––––– | x | –––––––––– | = number of O2 molecules |
| 31.998 g | 1 mol |
| 1 mol | 6.022 x 1023 | |||
| 50.0 g x | ––––––––– | x | –––––––––– | = number of I2 molecules |
| 253.8 g | 1 mol |
Avogadro's Principle Example
Example #10: 18.0 g of H2O is present. (a) How many oxygen atoms are present? (b) How many hydrogen atoms are present?
Solution:
1) Convert grams to moles:
18.0 g / 18.0 g/mol = 1.00 mol
2) Convert moles to molecules:
(1.00 mol) (6.02 x 1023 mol¯1) = 6.02 x 1023 molecules
3) Determine number of atoms of oxygen present:
(6.02 x 1023 molecules) (1 O atom / 1 H2O molecule) = 6.02 x 1023 O atoms
4) Determine number of atoms of hydrogen present:
(6.02 x 1023 molecules) (2 H atoms / 1 H2O molecule) = 1.20 x 1024 H atoms (to three sig figs)
Notice that there is an additional step (as seen in step 3 for O and step 4 for H). You multiply the number of molecules times how many of that atom are present in the molecule. In one molecule of H2O, there are 2 atoms of H and 1 atom of O.
Sometimes, you will be asked for the total atoms present in the sample. Do it this way:
(6.02 x 1023 molecules) (3 atoms/molecule) = 1.81 x 1024 atoms (to three sig figs)
The 3 represents the total atoms in one molecule of water: one O atom and two H atoms.
Example #11: Which of the following contains the greatest number of hydrogen atoms?
(a) 1 mol of C6H12O6
(b) 2 mol of (NH4)2CO3
(c) 4 mol of H2O
(d) 5 mol of CH3COOH
Solution:
1) Each mole of molecules contains N number of molecules, where N equals Avogadro's Number. How many molecules are in each answer:
(a) 1 x N = N
(b) 2 x N = 2N
(c) 4 x N = 4N
(d) N x 5 = 5N
2) Each N times the number of hydrogen atoms in a formula equals the total number of hydrogen atoms in the sample:
(a) N x 12 = 12N(b) 2N x 8 = 16N
(c) 4N x 2 = 8N
(d) 5N x 4 = 20N
(d) is the answer.
Example #12: How many oxygen atoms are in 27.2 L of N2O5 at STP?
Solution:
1) Given STP, we can use molar volume:
27.2 L / 22.414 L/mol = 1.21353 mol
2) There are five moles of O atoms in one mole of N2O5:
(1.21353 mol N2O5) (5 mol O / 1 mol N2O5) = 6.06765 mol O
3) Use Avogadro's Number:
(6.06765 mol O) (6.022 x 1023 atoms O / mole O) = 3.65 x 1024 atoms O (to three sig figs)
Example #13: How many carbon atoms are in 0.850 mol of acetaminophen, C8H9NO2?
Solution:
1) There are 8 moles of C in every mole of acetaminophen:
(0.850 mol C8H9NO2) (8 mol C / mol C8H9NO2) = 6.80 mol C
2) Use Avogadro's Number:
(6.80 mol C) (6.022 x 1023 atoms C / mole C) = 4.09 x 1024 atoms C (to three sig figs)
Example #14: How many atoms are in a 0.460 g sample of elemental phosphorus?
Avogadro Real Life Example
Solution:
Phosphorus has the formula P4. (Not P!!)0.460 g / 123.896 g/mol = 0.00371279 mol
(6.022 x 1023 molecules/mol) (0.00371279 mol) = 2.23584 x 1021 molecules of P4
(2.23584 x 1021 molecules) (4 atoms/molecule) = 8.94 x 1021 atoms (to three sig figs)
Set up using dimensional analysis style:
| 1 mol | 6.022 x 1023 molecules | 4 atoms | ||||
| 0.460 g x | –––––––– | x | –––––––––––––––––– | x | ––––––––– | = 8.94 x 1021 atoms |
| 123.896 g | 1 mol | 1 molecule |
Example #15: Which contains the most atoms?
(a) 3.5 molecules of H2O
(b) 3.5 x 1022 molecules of N2
(c) 3.5 moles of CO
(d) 3.5 g of water
Solution:
The correct answer is (c). Now, some discussion about each answer choice.Choice (a): You can't have half of a molecule, so this answer should not be considered. Also, compare it to (b). Since (a) is much less than (b), (a) cannot ever be the answer to the most number of atoms.
Choice (b): this is a viable contender for the correct answer. Since there are two atoms per molecule, we have 7.0 x 1022 atoms. We continue to analyze the answer choices.
Choice (c): Use Avogadro's number (3.5 x 1023 mol¯1) and compare it to choice (b). You should be able to see, even without the 3.5 moles, choice (c) is already larger than choice (b). Especially when you consider that N2 and CO both have 2 atoms per molecule.
Choice (d): 3.5 g of water is significantly less that the 3.5 moles of choice (c). 3.5 / 18.0 equals a bit less that 0.2 moles of water.
Bonus Example: A sample of C3H8 has 2.96 x 1024 H atoms.
(a) How many carbon atoms does the sample contain?
(b) What is the total mass of the sample?
Solution to (a):
1) The ratio between C and H is 3 to 8, so this:
| 3 | y | |
| ––––––– | = | –––––––––––––––– |
| 8 | 2.96 x 1024 H atoms |
2) will tell us the number of carbon atoms present:
y = 1.11 x 1024 carbon atoms
3) By the way, the above ratio and proportion can also be written like this:
3 is to 8 as y is to 2.96 x 1024Be sure you understand that the two different ways to present the ratio and proportion mean the same thing.
Solution to (b) using hydrogen:
1) Determine the moles of C3H8 present.
2.96 x 1024 / 8 = 3.70 x 1023 molecules of C3H8
2) Divide by Avogadro's Number:
Avogadro's Law Example Problem
3.70 x 1023 / 6.022 x 1023 mol¯1 = 0.614414 mol <--- I'll keep some guard digits
3) Use the molar mass of C3H8:
Avogadro's Law Formula Example
0.614414 mol times 44.0962 g/mol = 27.1 g (to three sig figs)




